Abstract

Introduction: High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (double hit lymphoma [DHL]) and double expressor lymphoma (DEL; overexpression of MYC and BCL2 on IHC) are distinct subtypes of DLBCL with inferior responses to front- and later line chemoimmunotherapy (CIT) (Horn Blood 2013) and autologous stem cell transplantation (ASCT) (Herrera JCO 2017). However, CAR T-cell therapy (CART) leads to similar overall response rates (ORR) for both DHL and non-DHL (Neelapu NEJM 2017, Schuster NEJM 2019). The duration of response among high-risk subtypes is unknown with minimal data on CART survival outcomes. Therefore, we performed a multicenter retrospective analysis evaluating survival outcomes with CART and post-CART progression for (1) DHL versus (v) non-DHL patients (pts) and (2) DHL v DEL v other pts.
Methods: Adult pts with R/R aggressive B-cell non-Hodgkin lymphoma (B-NHL) treated with CD19-directed CART from 2015-21 across 13 US centers were identified (n=536). Histologies other than de novo DLBCL and transformed follicular lymphoma were excluded. Baseline characteristics were compared between cohorts with Pearson Chi-squared test and included: age, sex, histology, cell of origin (COO), IPI, LDH at apheresis, prior ASCT, bulky disease, lines of therapy (tx) pre-CART apheresis, primary refractory/relapse within 12 months (mon) of initial CIT, bridging tx (BT), CART on clinical trial, CART product, CRS and ICANS. Median progression free and overall survival (mPFS and mOS) were estimated via Kaplan-Meier method. Variables associated with PFS on univariate analysis (UVA) were incorporated into the Cox multivariable regression analysis (MVA) to determine impact of variables on CART PFS. A p-value <0.05 was significant.
Results:DHL v non-DHL
408 pts were included, 80 with DHL and 328 with non-DHL (exclusion reasons: histology, n=59; missing DHL status, n=69). 64% received axi-cel, 26% tisa-cel and 10% liso-cel. Clinical characteristics were similar except DHL vs non-DHL pts were more likely to have GCB COO (87% v 54%, p<0.001), primary refractory/relapse within 12 mon of initial CIT (84% vs 71%, p=0.03) and receive CAR-T in the 2nd line (20% vs 10.4%, p=0.02). Median follow-up (f/u) from time of CART was 17.7 mon in surviving pts. CART ORR was similar for DHL v non-DHL (ORR 69% v 66%, p=0.7; CR 49% v 48%, p=0.8) as were mPFS (7.5 v 6.2 mon, p=0.2) and mOS (NR vs 21 mon, p=0.6). On MVA, there was no difference in CART PFS for DHL pts (HR 0.81, 95% CI 0.5-1.3, p=0.4), though >2 lines of tx pre-apheresis, BT and elevated LDH at apheresis predicted inferior PFS on MVA (HR 1.6, 95% CI 1.2-2.2; HR 1.7, 95% CI 1.3-2.4; HR 1.5, 95% CI 1.1-2.1, p<0.01 for all).
In pts progressing post-CART (DHL n=35; non-DHL n=175), DHL pts had inferior mOS (2.7 v 6.0 mon, p=0.02) from time of CART progression. Nonetheless, DHL pts received tx at a similar rate as non-DHL pts post-CART progression (54.2% v 66.3%, p=0.2) and had similar median time to relapse post-CART (89 v 89 days, p=0.6).
DHL v DEL v other
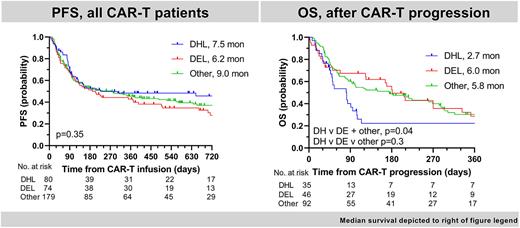
333 pts were included, 80 with DHL, 74 with DEL and 179 other pts (exclusion reasons: histology, n=59; missing DHL status, n=69; missing DEL status, n=75). Clinical characteristics were similar for DHL v DEL v other except for bulky disease at dx (31% v 40% v 21%, p=0.01), BT (38% v 61% v 45%, p=0.02) & GCB COO (87% v 58% v 55%, p<0.01). Median f/u from CART was 18.7 mon. CART ORR was similar for DHL v DEL v other (ORR 69% v 64% v 66%, p=0.8; CR 49% v 42% v 48%, p=0.6) as were mPFS (7.5 v 6.2 v 9.0 mon, p=0.35 Figure 1) and mOS (NR v 19.1 v 25.7 mon, p=0.8). On MVA there was no difference in CART PFS between cohorts (3-way p-value, p=0.3).
In pts progressing post-CART (DHL n=35; DEL n=46; other n=92), mOS from time of progression was worse for DHL v DE+other pts (2.7 v 5.9 mon, p=0.04; Figure 1), but not DH v DE v other pts (3-way p-value 0.3).
Conclusions: In the largest analysis that characterizes survival outcomes in R/R DHL and DEL treated with CART to date, mPFS and mOS of these high-risk pts mirrors that of other DLBCL pts. This represents a major therapeutic advance for this high-risk population with historically poor survival when treated with chemotherapy and supports early use of CART for these pts in the relapsed setting. DHL pts progressing post-CART have a dismal mOS of 2.7 mon, reflecting the urgent need for effective therapies post-CART failure. Trials exploring novel therapies post-CART should enrich for this population with more liberal inclusion criteria.
Disclosures
Shouse:Kite Pharma: Speakers Bureau; Beigene Inc USA: Honoraria. Torka:Lilly USA: Consultancy; Genentech: Consultancy; Targeted Oncology, Physician Education Review: Honoraria; ADC Therapeutics: Consultancy; Epizyme: Consultancy; TG Therapeutics: Consultancy. Moyo:Seattle Genetics: Consultancy. Romancik:AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Barta:Seagen: Honoraria; Affimed: Consultancy; Janssen: Other: Independent Data Monitoring Committee member; Daiichi Sankyo: Consultancy; Acrotech: Honoraria; Kyowa Kirin: Consultancy, Honoraria. Cohen:Kite Pharma/Gilead: Consultancy; Astrazeneca: Consultancy, Research Funding; Lilly Oncology/Eli Lilly: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; BMS/Celgene: Research Funding; Janssen: Consultancy; Genentech: Research Funding; Novartis: Research Funding; Takeda: Research Funding; Aptitude Health: Consultancy; HutchMed: Consultancy, Research Funding. Shah:Miltenyi Biotec: Consultancy, Research Funding; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; TG therapeutics: Consultancy; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Lilly Oncology: Consultancy, Honoraria; Epizyme: Consultancy; Kite Pharma: Consultancy. Hess:AstraZeneca: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy; ADC Therapeutics: Consultancy. Stephens:Karyopharm: Research Funding; Lilly: Consultancy; TG Therapeutics: Consultancy; Epizyme: Consultancy; Newave: Research Funding; Genentech: Consultancy; JUNO: Research Funding; Arqule: Research Funding; AbbVie: Consultancy; AstraZeneca: Consultancy; Novartis: Research Funding; Mingsight: Research Funding; CSL Behring: Consultancy; Acerta: Research Funding; Celgene: Consultancy; Beigene: Consultancy. Ma:BeiGene: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy; Janssen: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Research Funding; Juno: Research Funding; Loxo: Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding. Winter:Servier: Consultancy, Other: For Spouse; CVS/Caremark: Consultancy, Other: For Spouse; Astellas: Other: For Spouse, to University of Chicago, Research Funding; Rafael: Other: For Spouse, to University of Chicago, Research Funding; Forty Seven/Gilead: Other: For Spouse, to University of Chicago, Research Funding; Novartis: Consultancy, Other: for Spouse, to the University of Chicago, Research Funding; Daiichi Sankyo: Other: for Spouse, to the University of Chicago, Research Funding; Cellectis: Other: for Spouse, to the University of Chicago, Research Funding; Merck & Co., Inc.: Honoraria, Research Funding. Pro:Seattle Genetics: Honoraria. Moreira:CTI BioPharma: Consultancy; Ingenio Rx: Consultancy. Gordon:Ono Pharmaceuticals: Consultancy; Zylem: Current equity holder in private company, Current equity holder in publicly-traded company, Patents & Royalties: Patent on nanoparticles for lymphoma therapy; BMS: Research Funding; Janssen: Other: DSMB. Danilov:Astra Zeneca: Consultancy, Research Funding; Takeda Oncology: Research Funding; Nurix: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; MEI: Consultancy, Research Funding; Bristol-Meyers-Squibb: Consultancy, Research Funding; Cyclacel: Research Funding; Bayer Oncology: Research Funding; Genentech: Consultancy; Incyte: Consultancy; GSK: Consultancy; Morphosys: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy. Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board. Karmali:Karyopharm: Consultancy; Eusa: Consultancy; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; AstraZeneca: Other: Advisory Board, Speakers Bureau; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Takeda: Research Funding; BMS/Celgene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Other: Advisory Board; Genentech/Roche: Consultancy, Other: Advisory Board; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal